First (1st) consultation and prescription of combined oral contraceptive (COC) pill
Assessment of suitability of CHC (combined hormonal contraception) for an individual woman
Assessment of medical eligibility for CHC should include medical conditions, lifestyle factors and family medical history.
A drug history should identify:-
- any prescribed or non-prescribed drug that could affect the effectiveness of the contraceptive
- any prescribed or non-prescribed drug that could itself be affected by the contraceptive
- a recent, accurate blood pressure recording should be documented for all women prior to first CHC prescription
- BMI should be documented for all women prior to CHC prescription
- Pelvic examination is not required prior to initiation of CHC
- Breast examination, cervical screening, testing for thrombophilia, hyperlipidaemia or diabetes mellitus and liver function tests are not routinely required prior to initiation of CHC
- women for whom CHC is unsuitable should be offered alternative effective contraception
The HCP should obtain a history that includes the woman’s age, past and current medical conditions, smoking, drug history (prescription, non-prescription and herbal preparations) and family history of significant medical conditions. BMI and blood pressure should be recorded.
Assessment of medical eligibility for CHC
Medical history and lifestyle factors
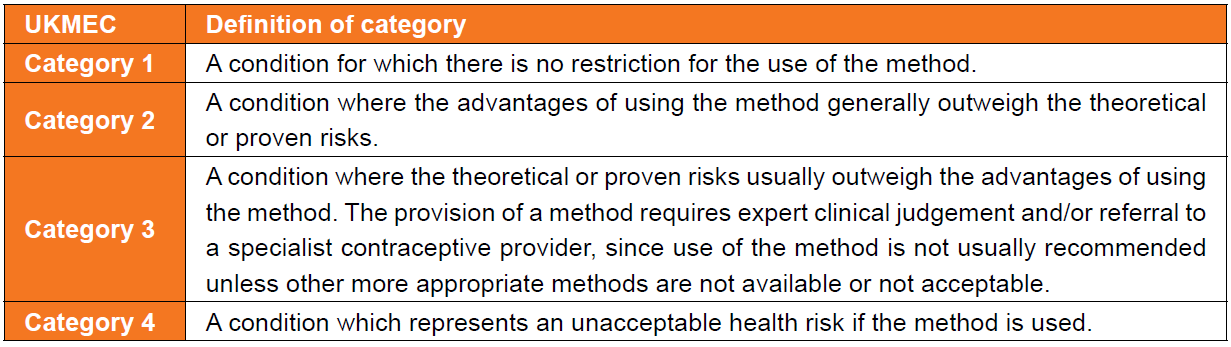
Definition of categories for the UK Medical Eligibility Criteria for Contraceptive Use
(UKMEC)
Specific attention should be given to enquiring about:
- thrombophilia or previous VTE
- ischaemic heart disease, stroke or transient ischaemic attack, peripheral vascular disease
- additional risk factors for venous or arterial thromboembolism (e.g. smoking, obesity, recent childbirth, immobility, hypertension, migraine, diabetes, hyperlipidaemia, antiphospholipid antibodies, arrhythmia, complicated congenital/valvular heart disease or cardiomyopathy)
- personal history of breast cancer/known breast cancer-related gene mutation
- hepatobiliary disease
- recent childbirth, current breastfeeding
Measurements and tests
- blood pressure and body mass index (BMI) should be documented for all women before prescription of CHC. The prescriber must be confident that measurements are recent and accurate
- Blood pressure:
- women with severe hypertension (systolic pressure ≥160 mmHg or diastolic pressure ≥100 mmHg) should not use CHC (UKMEC 4)
- women with less severe hypertension (systolic pressure 140–159 mmHg or diastolic pressure 90–99 mmHg), or with adequately controlled hypertension should not use CHC (UKMEC 3)
- blood pressure should therefore be evaluated before initiating CHC
- Weight (BMI):
- women with BMI <35 kg/m2 generally can use CHC (UKMEC 2)
- women with BMI >= 35 kg/m2 generally should not use CHC (UKMEC 3), although CHC may be prescribed by a specialist provider
- BMI should be documented before starting CHC. Baseline weight could additionally be helpful for monitoring any changes and counselling women who might be concerned about later weight change perceived to be associated with their contraceptive method
- Pelvic examination:
- a consultation regarding contraception may be used as an opportunity for health screening but screening should not be a condition for prescribing. Pelvic examination is not necessary before initiation of CHC because it does not affect the decision to prescribe or withhold hormonal contraception
- Clinical breast examination:
- although women with current breast cancer should not use CHC (UKMEC 4), screening asymptomatic women with a clinical breast examination before initiating CHC is not necessary because of the low prevalence of breast cancer among women of reproductive age
- although women with current breast cancer should not use CHC (UKMEC 4), screening asymptomatic women with a clinical breast examination before initiating CHC is not necessary because of the low prevalence of breast cancer among women of reproductive age
Assessment of factors that could affect contraceptive effectiveness
Drug interactions
- some medications could reduce the contraceptive effectiveness of CHC by induction of hepatic enzymes. Contraceptive hormones can affect the action of certain medications.
Malabsorption
- the effectiveness of COC (but not the CTP or CVR) could be reduced by malabsorption resulting from, for example, vomiting and severe diarrhoea
Key messages for women considering use of tailored combined hormonal contraception regimens
- the evidence from studies is that combined hormonal contraception (CHC) is as safe and at least as effective for contraception if it is taken as an extended or continuous regimen as it is when it is taken in a traditional 21/7 cycle
- a woman who is using CHC does not need to have a monthly withdrawal bleed to be healthy.
- there is no build-up of menstrual blood inside a woman who uses CHC for an extended time without a break; extended CHC use keeps the lining of the womb thin
- withdrawal bleeds during cyclical use of CHC have been reported by women who are pregnant; women should not consider monthly bleeds on CHC to be reassurance that they are not pregnant
- by using extended or continuous CHC the frequency of withdrawal bleeds and associated symptoms (e.g. headache, mood change) is reduced; this could be useful for women who have heavy or painful bleeding or problematic symptoms associated with the hormone-free interval (HFI)
- the ovaries start to become active during the traditional 7-day HFI. Fewer and/or shorter breaks in CHC use could mean that the risk of pregnancy could theoretically be lower with extended or continuous regimens than if a 7-day break is taken every month
- there can be irregular bleeding or spotting in the first few months of CHC use, particularly with extended or continuous regimens; this does not usually mean that there is any medical problem and it generally improves with time
- the evidence from studies is that using extended or continuous regimens of CHC does not affect the return of a woman’s fertility when she stops CHC.
Women using combined hormonal contraception: key indications for medical review
Key symptoms that should prompt women to seek urgent medical review
- calf pain, swelling and/or redness
- chest pain and/or breathlessness and/or coughing up blood
- loss of motor or sensory function
Key symptoms that should prompt women to seek medical review
- breast lump, unilateral nipple discharge, new nipple inversion, change in breast skin
- new onset migraine
- new onset sensory or motor symptoms in the hour preceding onset of migraine
- persistent unscheduled vaginal bleeding
New medical diagnoses that should prompt women to seek advice from their contraceptive provider (and review of the suitability of CHC)
- high blood pressure
- high body mass index (>35 kg/m2)
- migraine or migraine with aura
- deep vein thrombosis or pulmonary embolism
- blood clotting abnormality
- antiphospholipid antibodies
- angina, heart attack, stroke or peripheral vascular disease
- atrial fibrillation
- cardiomyopathy
- breast cancer or breast cancer gene mutation
- liver tumour
- symptomatic gallstones
For full details consult the reference below (1).
Reference:
- FSRH (July 2019). Combined Hormonal Contraception
- Faculty of Sexual & Reproductive Healthcare Clinical Guidance. Missed Pill Recommendations.Clinical Effectiveness Unit; May 2011.
Related pages
- Information women need to know in order to use the combined oral contraceptive pill appropriately
- Choice of combined oral contraceptive pill
- Follow-up of prescription of combined oral contraceptive pill
- Obesity and combined oral contraceptive
- Oral contraceptive pill (starting routines for COCP)
- General contraindications to combined oral hormonal contraceptive
Create an account to add page annotations
Add information to this page that would be handy to have on hand during a consultation, such as a web address or phone number. This information will always be displayed when you visit this page